- Laboratory dynamics
- notice
Laboratory dynamics
Breakthrough in CO2 Hydrogenation by Integration of Quantum Confinement and Alloy Effect
Recently,
the research group of Prof. Zeng has applied quantum confinement and alloy effect in CO2 hydrogenation to achieve remarkable catalytic activity by
fabricating RhW Nanocrystals as a catalyst. The d-band center and surface negative charge density generally
determine the adsorption and activation of CO2, thus serving as
important descriptors of the catalytic activity towards CO2 hydrogenation. Herein, researchers engineered the d-band center and negative charge density of Rh-based catalysts by
tuning their dimensions and introducing non-noble metals to form an
alloy. This work has been published on Nano Letters (Nano Lett. 2017, 17,
788-793)
with the title of “Integration of Quantum Confinement
and Alloy Effect to Modulate Electronic Properties of RhW Nanocrystals for
Improved Catalytic Performance toward CO2 Hydrogenation”. Master
Wenbo Zhang and Doctor Liangbing Wang contributed equally to this work.
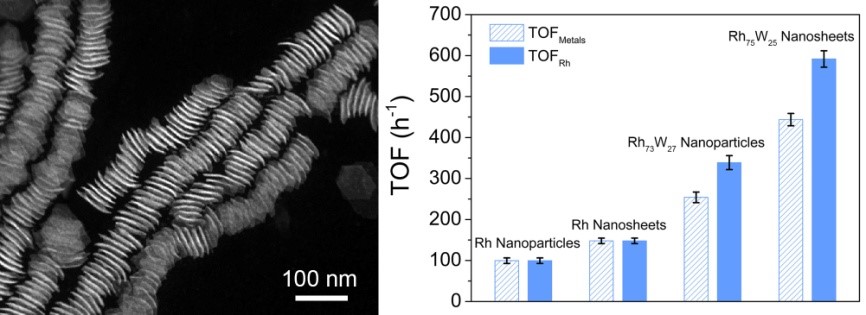
RhW nanocrystals and their catalytic performance
The fixation and reduction of CO2 into
useful chemicals and fuels have attracted tremendous interest to meet current energetic
and environmental demands. Considering the high stability of a CO2 molecule, activation of CO2 plays a pivotal role in the chemical transformation
of CO2. This process can be realized through heterogeneous catalysis
where the catalytic performance is largely determined by the electronic
properties of the surface. Based on theoretical studies, tuning the dimension
of nanostructures represents an effective strategy to engineer the surface
electronic properties by varying the spatial distribution of electrons.
Another strategy for electronic modification is to form an alloy by adding
another metal; charge transfer will then occur owing to the different electro negativities
of the constituent metals.
Herein, researchers combined these two strategies to
tune the electronic properties of Rh-based nanocrystals in order to enhance the
catalytic activity towards CO2 hydrogenation. During CO2 hydrogenation, RhW nanosheets exhibited remarkable catalytic activity with the
turnover frequency (TOF) number of 592 h-1, which was 5.9, 4.0, and
1.7 times as high as that of Rh nanoparticles, Rh nanosheets, and RhW
nanoparticles, respectively. Mechanistic studies reveal that the remarkable
activity of RhW nanosheets derives from the integration of quantum confinement
and alloy effect. Specifically, the quantum confinement in
one dimension shifts up the d-band
center of RhW nanosheets, strengthening the adsorption of CO2 relative to the nanoparticles. Moreover, the electron transfer from W to Rh
enables the accumulation of negative charges on surface Rh atoms in the case of
RhW nanosheets, benefiting the activation of CO2. The enhancement in
the adsorption and activation of CO2 for RhW nanosheets was directly
revealed by in-situ diffuse
reflectance infrared Fourier transform (DRIFT) spectra. This approach paves the
effective way to modulate the electronic properties of catalysts to achieve superior
catalytic performance.
This work was supported by MOST of China, the
National Natural Science Foundation of China, etc.
Publication
link: http://pubs.acs.org/doi/abs/10.1021/acs.nanolett.6b03967.

