Recently, the research group of Prof. Zeng has demonstrated at the atomic level how hydrophilicity of SiC quantum dots (QDs) boosts their catalytic activity in CO2 hydrogenation via the involvement of hydroxyl species. This work has been published on Chem with the title of “Molecular-Level Insight into How Hydroxyl Groups Boost Catalytic Activity in CO2 Hydrogenation into Methanol” [Chem 2018, DOI: 10.1016/j.chempr.2018.01.019]. Doctor Yuhan Peng, Doctor Liangbing Wang and Associate Researcher Qiquan Luo contributed equally.
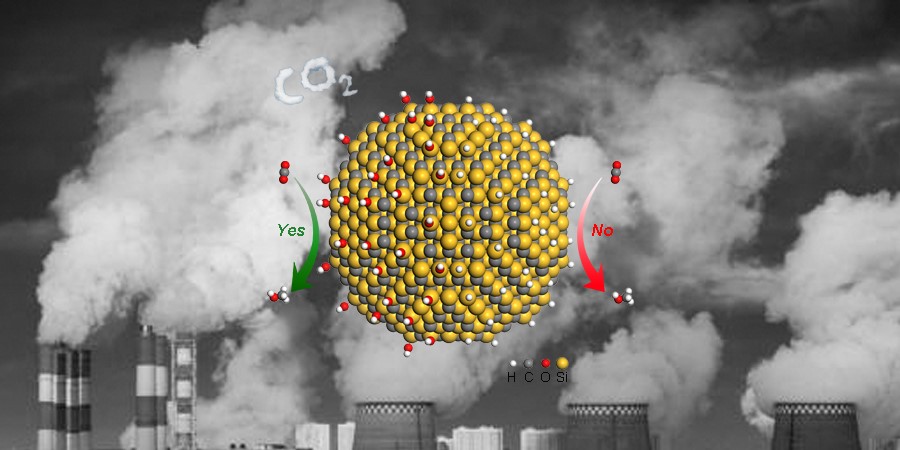
Illustration of CO2 hydrogenation over SiC QDs
Since the catalysis proceeds on the surface of catalysts, engineering their surface properties serves as a viable access to manipulate the catalytic activity, selectivity, and stability. A pivotal intrinsic surface property is hydrophilicity, the affinity of a material for water molecules. The most common understanding of the effect of hydrophilicity on catalytic properties is based on regulating the concentration of reactants accessible to active sites. Specifically, hydrophilic surface is able to enrich hydrophilic reactants such as alcohols around the active sites, whereas hydrophobic surface facilitates the accumulation of hydrophobic reactants including esters and aromatic ketones. However, little attention has been focused on the atomic-level insight into how hydrophilicity regulates catalytic properties. Exploring the influence of hydrophilicity on catalytic performance at the atomic level has great potential to offer a guideline for developing highly efficient catalysts and deepen the mechanistic understanding of heterogeneous catalysis, but still remains as a grand challenge.
The researchers found in CO2 hydrogenation, the mass activity of hydrophilic SiC QDs reached 169.5 mmol g-1 h-1 under 32 bar of CO2/H2 mixed gas (CO2:H2 = 1:3) at 150 oC, more than three orders of magnitude higher than that of hydrophobic bulk SiC. The activation energy for SiC QDs was 48.6 KJ mol-1, about half of that (94.7 KJ mol-1) for bulk SiC. According to mechanistic studies, the enhanced activity of SiC QDs was closely correlated with their hydrophilicity. Specifically, the surface hydroxyl species on SiC QDs directly participated in CO2 hydrogenation through the addition of H atoms in hydroxyl groups into CO2 to form HCOO* as the intermediate. In addition, the unique reaction path induces the decrease of energy barrier for the formation of HCOO*, facilitating the activation of CO2 and accordingly improving the activity for CO2 hydrogenation. More importantly, their understanding on surface hydrophilicity offers a guideline for the development of efficient catalysts toward CO2 hydrogenation. Metal hydroxides and layered double hydroxides were determined as highly active catalysts, exhibiting more than one-order-of-magnitude enhancement in mass activity relative to their metal oxide counterparts.
This work was supported by MOST of China, and the National Natural Science Foundation of China.

