Recently, the research group of Prof. Zeng has investigated the active phase of cobalt (Co)-based catalysts in CO2 hydrogenation. They have incorporated N atoms into Co catalysts to fabricate Co4N catalysts. Based on in-situ mechanistic studies, they found that Co4NHx served as the active phase during CO2 hydrogenation. This work has been published on Nature Energy with the title of “Incorporating nitrogen atoms into cobalt nanosheets as a strategy to boost catalytic activity toward CO2 hydrogenation” [Nature Energy 2017, DOI: 10.1038/s41560-017-0015-x].
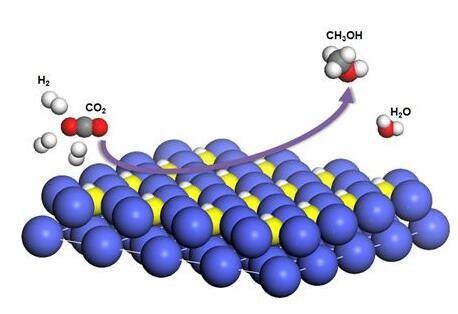
Illustration of CO2 hydrogenation over Co4N catalysts
With the rapid development of technology and industrialization, the demand for energy has been increasing year by year. Because of the heavy use of fossil fuels, the energy and environmental crisis have compelled human society to seek for renewable energy sources. The hydrogenation of CO2 into fuels and valuable chemicals has emerged as an effective strategy to both mitigate the green house effect and achieve a sustainable carbon cycle. CO2 hydrogenation also serves as an efficient approach to store hydrogen which derives from electrolytic production.
Although the chemical transformation of CO2 has been achieved in industry, the reaction requires high pressure (50-100 bar) and elevated temperature (200-300 ℃) because of the chemical inertness of CO2. The past decades have witnessed the development of strategies to improve the activity of non-noble metal catalysts towards CO2 hydrogenation. Up to now, the investigation of the active phase of non-noble catalysts in CO2 hydrogenation is still at the rudimentary stage.
The researchers incorporated N atoms into Co catalysts to synthesize Co4N catalysts. In CO2 hydrogenation, Co4N catalysts exhibited a turnover frequency (TOF) of 25.6 h-1 under 32 bar at 150 ℃, which was 64 times as high as that of Co catalysts. The activation energy for Co4N catalysts was 43.3 kJ mol-1, less than half of that (91.4 kJ mol-1) for Co catalysts. Further mechanistic studies revealed that Co4NHx was formed by adsorbing H atoms on N atoms in Co4N nanosheets in the presence of H2. The amido-hydrogen atoms in Co4NHx directly added to CO2 to form HCOO* as the intermediates. In addition, the adsorbed H2O* was found to activate the amido-hydrogen atoms via the interaction of hydrogen bonds, which facilitated the hydrogenation process. This work not only provides a way to engineer efficient, low-cost catalysts for CO2 hydrogenation via the incorporation of N atoms, but also advances our understanding of active phase of Co-based catalysts.
This work was supported by MOST of China, and the National Natural Science Foundation of China.
Source: https://www.nature.com/articles/s41560-017-0015-x.

