- Laboratory dynamics
- notice
Laboratory dynamics
Recently, the research group of Prof. Jie Zeng cooperated with Prof. Guoxiong Wang from Dalian Institute of Chemical Physics have made a breakthrough in CO2 electrochemical reduction, the researchers introduce the oxygen vacancies into electrocatalysts with electronic-rich surface to enhance the activity and selectivity towards CO2 electrochemical reduction. This work as a hot paper has been published on Angewandte Chemie international Edition (Angew. Chem. Int. Ed. 2018, 57, 6054-6059) with the title of “Oxygen Vacancies in ZnO Nanosheets Enhance CO2 Electrochemical Reduction into CO”. Dr. Zhigang Geng, graduate student Xiangdong Kong and Weiwei Chen contributed equally to this work.
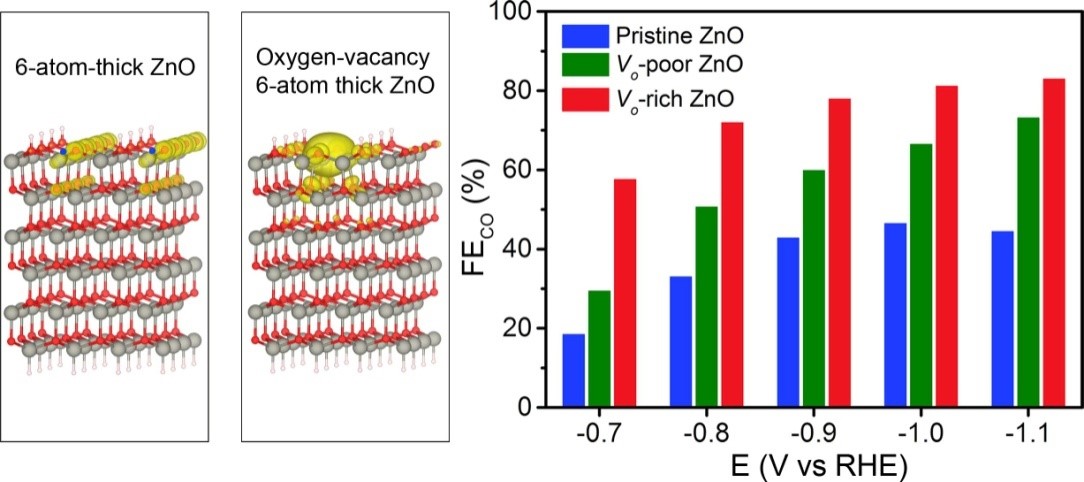
Calculated DOS of ZnO slab and ZnO slab with oxygen vacancy. Faradaic Efficiency for CO production on the three ZnO nanosheets at selected potentials.
Due to the high thermodynamic stability of CO2, the standard potential of CO2/ CO2- couple is as negative as -1.9 V versus reversible hydrogen electrode (vs RHE) in water for the formation of highly energetic CO2 anion radicals. As such,the effective activation of CO2 plays a key role in CO2 electrochemical reduction. The electron transfer to CO2 is generally considered as the critical step during the activation of CO2. Therefore, it is especially important to rationally design a highly active and robust electrocatalyst which possesses assistant electron-donation centers to capture CO2 molecules and activate the stable C=O non-polar bonds. The introduction of oxygen vacancies provides a powerful method to promote CO2 adsorption and activation due to surface electronic-rich.
Herein, we developed a versatile route to the introduction of oxygen vacancies into ZnO nanosheets by H2 plasma treatment. During the CO2 reduction reaction, ZnO nanosheets with rich oxygen vacancies exhibited a current density of -16.1 mA cm-2 with the Faradaic efficiency of 83% for CO production. Based on the density functional theory calculations, the introduction of oxygen vacancies increased the charge density of ZnO around the valence band maximum, resulting in the enhanced activation of CO2. Mechanistic study further revealed that the enhancement of CO production by introducting oxygen vacancies into ZnO nanosheets originated from the promoted binding strength of CO2 and the facilitated CO2 activation. This work would provide a guideline for the rational design of novel and highly efficient catalysts towards CO2 electrochemical reduction by regulating electronic properties of catalysts.
This work was supported by CAS, MOST of China, and National Natural Science Foundation of China.
Source: https://onlinelibrary.wiley.com/doi/abs/10.1002/anie.201711255

