- Laboratory dynamics
- notice
Laboratory dynamics
Recently, the research group of Prof. Jie Zeng has made great progress in revealing the strain effect in electrochemical reduction of CO2 through the collaboration with Prof. Jinlong Yang from School of Chemistry and Materials Science. By using Pd octahedra and icosahedra as a well-defined platform, researchers successfully demonstrate the internal correlation between surface strain and catalytic performance in the electrochemical reduction of CO2. This work has been published on Angewandte Chemie International Edition with the title of “Understanding of Strain Effect in Electrochemical Reduction of CO2: Using Pd Nanostructures as an Ideal Platform” [Angew. Chem. Int. Ed. 2017, 56, 3594]. Postdoctoral researcher Dr. Hongwen Huang, graduate students Huanhuan Jia, and Zhao Liu contributed equally to this work.
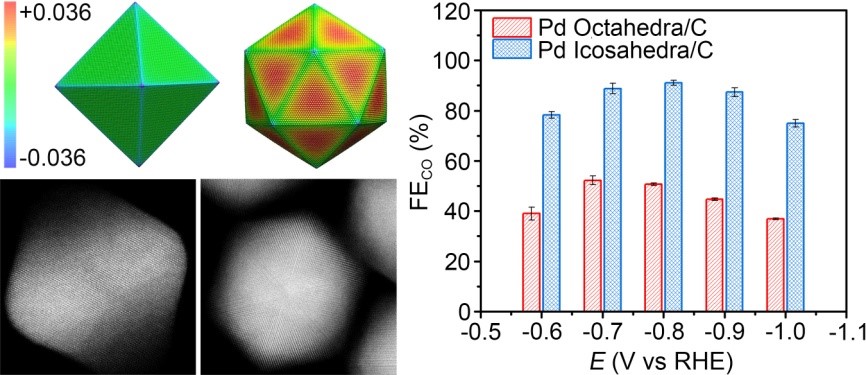
Pd nanostructures and their catalytic performance in electrochemical reduction of CO2
The increasing consumption of fossil fuels has led to dramatically rising atmospheric CO2 levels, which is primarily responsible for global warming. As a promising solution to this climate issue, electrochemical reduction of CO2 can help convert CO2 into value-added chemical feedstocks in a sustainable fashion, which further helps relax the severe burden of energetic sources. Moreover, powered by solar and other sources of renewable electricity, the electrochemical reduction of CO2 provides a strategy to store these intermittent sources of energy into high-energy chemicals using hydrogen source obtained from electrolysis of water. Unluckily, limited from the lack of excellent electrocatalysts, this technology is still difficult to be commercialized on a large scale, which can be overcame by deepening the understanding of the correlations between structure and properties.
Tuning the surface strain of heterogeneous catalysts represents a powerful strategy to engineer their catalytic properties by altering the electronic structures. However, a clear and systematic understanding of strain effect in electrochemical reduction of CO2 is still lacking, mainly owing to the difficulty in distinguishing the strain effect from the electronic effect. In this contribution, we design an ideal platform based on Pd octahedra and icosahedra with the same size, facet, and capping agent to explore the strain effect on the activity in CO2 electrochemical reduction. The electrochemical measurements indicate that the Pd icosahedra/C catalyst shows a maximum Faradaic efficiency for CO production of 91.1% at -0.8 V versus reversible hydrogen electrode (vs. RHE), which is 1.7-fold higher than the maximum Faradaic efficiency of Pd octahedra/C catalyst at -0.7 V (vs. RHE). The combination of molecular dynamics simulations and density functional theory calculations reveals that the improvement in catalytic activity stems from the tensile strain on the surface of Pd icosahedra, which shifts up the d-band center and thus strengthens the adsorption of key intermediate COOH*. This work not only provides an in-depth understanding of the strain effect but also offers an effective knob to tune the catalytic properties for electrochemical reduction of CO2.
This work was supported by CAS, MOST of China, National Natural Science Foundation of China, and China Postdoctoral Science Foundation.
Source: http://onlinelibrary.wiley.com/doi/10.1002/anie.201612617/abstract.

